![skip navigation [skip navigation]](/Images/dot_t.gif)
 Spectral Lines
Spectral Lines
The ASD database provides access to transition data for atoms and atomic ions.
For more information on the Lines data accessible by the database consult the
Introduction to and Contents of the ASD Database.
This section starts with the description of the input parameters of the Lines Search Form. For the description of the
output, either in tabular or graphical form, see the Lines Output section.
The ASD Lines Search Form, referred to as the "Lines Form," provides
access to transition data for atoms and ions, either in tabular or graphical form.
Tabular output is available for wavelengths (or wavenumbers, or photon energies, or frequencies), relative intensities, radiative
transition probabilities and related quantities, as well as energy level classifications
and bibliographic references. Graphical output is available in the forms of Grotrian
diagrams, line identification plots, and Saha-LTE spectrum plots. For some spectra, there
is also graphical information on dependences of certain line intensity ratios on
electron density and/or temperature in the emitting plasma. This information can be
useful for plasma diagnostics.
When loaded in the browser, the Lines Search Form displays only the Main search parameters block
and the Advanced Settings block below it.
At the bottom of the Main search parameters block, there are buttons "Reset Input," "Retrieve Data,"
"Show Graphical Options," and
"Hide Advanced Settings."
When a "Show ..." button is clicked, the corresponding block of input options
appears on the screen, and the button changes its name to "Hide ..."
Conversely, clicking a "Hide ..." button causes the corresponding block of input options disappear,
and the button changes its name to "Show ..."
The Lines Form prompts the user for the following pieces of information:
- Main search parameters
- Spectra of interest (e.g.,
Na I, Na II, Mg I-III, Mn-Co Ar-like).
- Primary quantity of interest: wavelength (default), wavenumber, photon energy, or frequency; selected from a pulldown menu in the Lines Form.
- Lower and upper limits of the range of the primary quantity
- Units for the quantity of interest: angstroms (Å), nanometers (nm; default for wavelengths), micrometers (μm);
cm−1 (the only choice for wavenumbers); eV (default for photon energies), Ry, Hartree; GHz (the only choice for frequencies).
The units are selected from a pulldown menu in the Lines Form. The same units apply both to the output quantity
and to the lower/upper limits of the quantity range.
- Graphical Output Options
- Advanced Settings
For the description of the output, see the Lines Output section.
 Main Search Parameters
Main Search Parameters
At a minimum, the user must enter spectra of interest
(e.g., H I) and then press the [Enter]
key or click "Retrieve Data". This will result in a tabular output page with
transition data. Alternatively, the user can click on the "Make Grotrian Diagram"
button to produce an interactive graphical diagram. The Dynamic Plots
require some additional settings, such as wavelength limits and
units.
 Selecting Spectra for Line Searches
Selecting Spectra for Line Searches
On the Lines Form, to specify an element, simply enter the element symbol (e.g.,
Fe). Element symbols and Roman numerals need not be capitalized.
Multiple elements are separated by a semicolon. To indicate the spectrum of a given
element, enter either a Roman numeral or an Arabic numeral after the element
name. The Roman numerals must be separated from the element symbol by a space.
(Note: Fe I = Fe0+,
Fe II = Fe1+, etc.). Alternatively, the
spectrum may be specified by the name of its isoelectronic sequence (e.g.,
Si Li-like = Si XII, Si Na-like = Si IV).
The absence of a Roman or Arabic numeral
or an isoelectronic sequence name after an element symbol indicates all stages of
ionization. Spectra of the same element are separated by a comma, while
spectra of different elements are separated by a semicolon. A range of spectra is indicated
by using a hyphen between stages of ionization or between names of isoelectronic sequences.
If the user has not provided enough information to specify spectra, an error
message will be displayed.
Examples of Spectral Notation (Case Insensitive)
| Na I |
|
Neutral sodium |
| na 0 |
|
Neutral sodium |
| Na I; Fe I |
|
Neutral sodium and neutral iron |
| Fe I-III |
|
Fe, ionization stages one, two, and three |
| Fe I-III,V |
|
Fe, ionization stages one, two, three, and five |
| Fe |
|
All ionization stages of iron |
| 198Hg I |
|
Neutral isotope 198 of mercury |
| C I; N II; O III |
|
List of spectra specifying neutral carbon, nitrogen II, and
oxygen III. |
| C-O C-like |
|
List of carbon-like spectra of all elements between carbon and oxygen (produces the same results as the previous example). |
| C-O I-III |
|
List of spectra of neutral, singly-ionized, and doubly-ionized elements between carbon and oxygen. |
| C-N I-II; Ne IV-V |
|
List of spectra specifying C I, C II, N I, N II, Ne IV, and Ne V. |
| Mg He-like-Li-like; Al Li-like |
|
List of spectra specifying Mg X, Mg XI, and Al XI. |
 Lower and upper limits of the wavelength/wavenumber/photon energy/frequency range
Lower and upper limits of the wavelength/wavenumber/photon energy/frequency range
By default, the Search Form prompts the user to enter the lower and upper limits of line wavelengths in the units
selected in the "Wavelength Units" menu. The user can change the primary quantity of interest from the default
"Wavelength" to "Wavenumber" or "Photon Energy" or "Frequency"
by selecting a corresponding option in the "Search for" pull-down menu. Change of the choice in this menu results in
corresponding changes in the name and contents of the "Units" menu and in available options in the
Advanced Settings section of the Search Form.
The optional input in the "Lower" and "Upper" input boxes is expected to be
in units chosen in the "Units" menu. Both the lower and upper limits can be left blank if the
Spectra box is non-blank and no Graphical Output Options are set. Otherwise, either one or both of them can be blank.
Note that the units set in the drop-down menu in the Main Parameters section apply not only to the
limits of the promary quantity search range, but also to the output. By default, the wavelengths are included in the output. The user
can change the choice of the columns displayed in the output and set a number of other options (e.g., whether the
wavelengths are in standard air or in vacuum) in the Advanced Settings section of the Search Form. These settings
will be displayed if the user clicks on the "Show Advanced Settings" button.
For the description of the tabular output of the Lines Form, see the Output Line Tables section.
 Graphical Output Options
Graphical Output Options
There are two sets of graphical output options in the Lines Search:
To show these graphical options, the user must click on the "Show Graphical Options" button in the Main Parameters section.
The contents and use of the graphical output are explained in the Graphical Output section.
 Dynamic Plot Options
Dynamic Plot Options
These options allow graphical display of three types of
dynamically created plots, i.e., line identification plots, spectrum image plots, and Saha-LTE
(local thermodynamic equilibrium) plasma emission plots. The first two types of plots are
created as PDF files that may require appropriate software (e.g., Adobe
Acrobat Reader or xpdf) for graph display. See the Graphical Output
section for the details on the output.
- Line Identification Plot
Selecting this option would produce a PDF file showing positions of all spectral lines of the chosen
Spectra within the chosen wavelength/wavenumber/photon energy/frequency range.
The contents and use of the Line Identification Plots are explained in the corresponding Output section.
- Spectrum Image Plot
This is an experimental feature introduced in ASD v.5.11 in 2023, and its operation has a limited flexibility.
For example, it presently works only if the search is restricted to a single atom or ion, and only if the
search is made for wavelengths in the units of nm, within the range between 380 nm and 780 nm. The purpose of the plot is to simulate an
appearance of the spectrum produced by a slit spectrograph in a visible range, so that the color of each spectral line roughly corresponds
to human percention of light at the line's wavelength, and its intensity roughly corresponds to intensities listed in the tabular output of ASD.
The lines listed in ASD without numerical intensity values are assigned small non-zero intensities to make them visible in the image.
The plot is displayed as an image at the bottom of the lines tabular output.
- Saha-LTE Spectrum
Selecting this option would produce a PDF file with a plot showing line emission
from an optically thin plasma having the chosen values of electron
temperature and electron density. For this plot, both the lower and upper limits of the wavelength/wavenumber/photon energy/frequency range
are mandatory parameters.
The plasma emission intensities are calculated in
arbitrary units. These units can be chosen from one of the two options:
1) energy flux units (the default choice) or
2) photon count units.
If only one ion/atom is chosen for plot generation, then
the populations of the energy levels are calculated according to the
Boltzmann formula Nk =
N0/g0·gk·exp(−Ek/Te)
where Nk is the level population, N0 is
the population of the ground state level, gk and
g0 are the statistical weights of the levels,
Ek is the energy of the level with respect to the ground
state in eV, and Te is the electron temperature in eV to be
entered in the text field. In this case the electron density
Ne is not required to be entered.
If several ions/atoms are chosen for plot generation (e.g., "C I-V" is entered in
the Spectra input box), then first the ionization distribution between
different ions is calculated according to the Saha formula (see, e.g.,
H.R. Griem, Principles of Plasma Spectroscopy, 1997), and then within
each ion, the populations are calculated using the Boltzmann distribution
formula. In this case both the electron temperature and electron density are
mandatory parameters.
The total number of photons emitted per second per unit volume of plasma in each radiative transition
is calculated by multiplying the population density of the upper level, Nk,
by the rate of spontaneous radiative decay, Aki (a.k.a. transition probability
or Einstein A-coefficient), from the upper level k to the lower level i of the transition.
If the user requested the intensity scale to be in terms of energy flux, the photon emission rate
is multiplied by the photon energy, Ek − Ei (in units
of cm−1 to calculate the emitted energy flux per unit plasma volume.
The spectrum may be convoluted with the Doppler (Gaussian) line profile for
each of the spectral lines. To do that, the user must check the Doppler-broadened spectrum box
in the Lines Search Form and (optionally) specify the Ion Temperature (in eV) corresponding to the desired broadening.
In practice, experimental spectra are broadened by many different mechanisms, e.g., instrumental broadening.
To produce a synthetic spectrum resembling experimental ones, the user may need to specify an unphysically large
Ion Temperature. The only meaning of this parameter is the broadening it produces.
By default (if no Ion Temperature was specified), the entered electron temperature
Te is used for calculation of the line width parameter. An
ion temperature Ti may be entered if
Ti ≠ Te.
If a Doppler-broadened spectrum is requested by the user, the spectrum will be calculated on a grid with the step
size defined by the total number of spectral lines of the selected spectra in the requested range and by the
broadening of each spectral line (i.e., Ion Temperature). The greater the number of lines, the greater is the
number of grid points required. The greater is the Ion Temperature, the lesser grid points are required. To reduce
the load on our database servers, we are limiting the total number of data points in the spectrum grid. If the
user's selection requires too many grid points, the ASD codes will choose a greater Ion Temperature corresponding
to the maximum allowed grid points, and a warning about it will be displayed in the output.
If several chemical elements are involved in the string entered in the Spectra input box,
an additional form will appear, requesting the user to enter the percentage abundances of each element in the mixture.
Such an additional form will also appear if the requested Spectra contain hydrogen or deuterium. For these spectra,
the user must specify whether the resolved fine structure or configuration-average wavelengths should be used for their
lines. This is due to the special character of hydrogen and deuterium spectra. In these spectra, the widths of the fine
structure of energy levels are exceptionally small and thus, the fine structure is usually unresolved in experimental
spectra recorded with low or moderate resolution. However, due to the fundamental importance of these spectra in physics,
many studies of them are made with exceptionaly high resolution. To accommodate the needs of both low- and high-resolution
spectroscopy, ASD contains two overlapping subsets of energy level and spectral line data for both H and D in the same
data sets. For calculation of simulated spectra of H and D, only one subset of these data, either low- or high-resolution,
must be selected (otherwise, the partition functions would be doubled, and
the calculated intensities would be too large). For other elements, ASD does not have both these types of energy levels and
wavelengths mixed in the tables, so a prompt for this choice will not appear.
The contents of the output Saha-LTE plots and tables accompanying them are explained in the corresponding
Output section.
 Java Grotrian Diagrams
Java Grotrian Diagrams
The output content and features of interactive Grotrian diagrams are explained in the Plotting Grotrian diagrams
section. The Grotrian Diagram can be displayed only for a spectrum of single atom or ion. Any restrictions applied to the selected data set, e.g.,
the wavelength/wavenumber/photon energy/frequency range, will be applied to selection of the data displayed in the diagram.
 Advanced Settings
Advanced Settings
The options shown in this block of the Lines Form are divided into three groups:
The following options apply to all lines and levels searches
and are collectively referred to as output options.
- Choice of display using an HTML table, an ASCII fixed-column-width table, a CSV or tab-delimted data file.
- Choice of removing Javascript tags in the output (convenient for an ASCII table when saving the output into a file)
- Energy level units. The user may choose between cm−1 (default), eV, Rydberg, Hartree (=2×Rydberg), or GHz.
- Choice of viewing the (scrollable) data all at once, or one page at a time.
- Choice of page size. The number of lines displayed on each page
of the output may be modified so that they would fit the user's screen size.
- Output ordering. The output can be sorted according to either wavelength
or multiplet order. The multiplet order is available if only one
ion or atom is specified in the Spectra box.
Wavelength ordering
All spectra are intermingled according to wavelength ordering. A spectrum must
be provided if no wavelength range is indicated.
Multiplet ordering
Multiplets are transitions that share the same term and configuration.
Multiplets have been ordered in the transition probability compilations
according to energies and g values of the lower and upper levels,
and have been assigned arbitrary multiplet numbers that reflect this order.
To view multiplet-ordered data, the user must select the "Multiplet ordering"
radio box.
In some cases, a multiplet is missing from the numbered list. In general, this
is because some property of a compiled wavelength or level involved is
consistent with other more recent compilations, such as the NIST energy level
data.
Only the lines with energy level classification are displayed in the
multiplet-ordered output, and therefore the total number of lines shown at the
top of the page may be different for wavelength and multiplet orderings.
The default is to display output in its entirety as an HTML formatted table.
By default, the levels are displayed in cm−1.
For instructions on how to modify options associated with viewing data, refer
to the section options for viewing data.
 Optional Search Criteria
Optional Search Criteria
The following search criteria may be specified:
- Maximum lower level energy.
- Maximum upper level energy.
- Preference of whether the transition strength bounds will apply to
Aki (default), fik, S, or
log(gf) values.
- Minimum and/or maximum transition strength.
- Accuracy minimum for Aki, fik, S, or log(gf).
- Relative intensity minimum.
 Additional Search Criteria
Additional Search Criteria
The following options apply to all line searches and are
collectively referred to as additional search criteria options.
- Line selection options:
The default is to display all stored lines of data meeting the search criteria,
regardless of whether the lines contain transition probability data,
energy level classifications, or plasma-diagnostics data,
and to not display any additionally available possible Ritz transitions.
- Choice of display of the plasma-diagnostics data:
Plasma diagnostics data were added to ASD when version 5.3 was released. For the list of spectra that
include these data, refer to the Version History (search for the word "diagnostics").
To include the diagnostics data in the Lines output, check the corresponding checkbox.
- Choice of bibliographic information output:
If the corresponding checkbox is checked, the bibliographic
references for transition probabilities (TP) and spectral lines will be
shown in two separate columns.
- Choice of display of wavelength data:
- Observed wavelength
- Ritz wavelength
- The default is to suppress the display of the "observed-Ritz"
column of data. Checking the "observed-Ritz" option will cause
that column of data to be generated in the output.
- Choice of wavelength type:
The default is to display wavelengths in:
Vacuum (< 200 nm), Air (200 nm to 2,000 nm), Vacuum (> 2,000 nm).
For wavelength ordered output, the table headings for the wavelength
columns change as needed to reflect the change in wavelength type.
For multiplet ordered output, the type of the output value (wavelength in air or vacuum or wavenumber,
which is always in vacuum) and the measurement units are specified in a separate column next to the
values.
Alternative choices are:
- Vacuum (< 200 nm), Air (200 nm to 1,000 nm), Wavenumber (> 1,000 nm)
- Vacuum (< 1,000 nm), Wavenumber (> 1,000 nm)
- Vacuum (< 200 nm), Air (200 nm to 2,000 nm), Vacuum (> 2,000 nm)
- Vacuum (all wavelengths)
- Vacuum (< 185 nm), Air ( (> 185 nm))
- Wavenumber (all wavelengths)
Note that the formula we use for the refractive index of air was experimentally verified in the wavelength
range between 185 nm and 1690 nm. The refractive index of air is not known for wavelengths outside of this range, where
air strongly absorbs all radiation. For this reason, wavelengths shorter than 185 nm and 1690 nm cannot be displayed
in air. For wavelengths >1690 nm, output of wavelengths in air is allowed, because the formula for the refractive index
of air behaves smoothly in this range. However, the users should be cautious when using such wavelengths, as their validity is
uncertain.
To change the default, the user simply needs to click on one of the
radio buttons. The user will also need to check appropriate checkboxes
if individual columns of wavelength information are desired:
- Choice of display of transition strength information. The default is to
display the following columns of data:
- Aki,
- Accuracy,
- Relative intensity.
By default, the Aki (or gkAki)
are displayed in units of s−1. They can be displayed in units
of 108 s−1 if a proper checkbox is checked.
To suppress display of the Relative Intensity data column, the
corresponding checkbox can be unchecked.
Although the fik, S, and log(gf) values are not
displayed by default, if the corresponding checkboxes are clicked, then those data values will be displayed.
- Choice of transition type:
By default, both electric dipole-allowed (E1) and forbidden (M1, E2, M2,...) transitions are displayed in the output.
To display only allowed or only forbidden transitions, a user must uncheck the checkbox corresponding to
the unwanted type of transitions.
- Choice of level information: For lines output, the default is to display:
- Configurations,
- Terms,
- Energies, and
- J values.
To suppress display of the information listed above, the corresponding
checkbox can be unchecked.
In addition to displaying the lines stored in the database, the user can choose to display Ritz wavelengths of all possible electric dipole (E1)
transitions between the energy levels available in the database. The set of these transitions is defined by strict selection
rules: the levels must be of opposite parities, and the total angular momentum (J) must change by no more than ±1;
transitions between the levels that both have J = 0 are also forbidden and are not included in the list.
In addition to these strict rules, the following two categories of transitions are also omitted from the ASD output:
- Thansitions involving levels corresponding to unresolved terms consisting of several closely lying fine-structure levels with different J values.
These levels may have a blank J value in the database or a comma-separated list of several J values.
- Transitions between levels differing by less than 2000 cm−1.
Even with these limitations, the total number of E1 transitions that can potentially be displayed amounts to a few million. For this reason, this option
can be used only for a single spectrum specified in the Spectra input box.
The Ritz transitions do not have any data on observed wavelength, intensity, or transition probability. For this reason, selecting this option is ignored when the user
chooses to display only the observed lines, or only lines with transition probabilites, or selects the Multiplet ordering option for the output,
or sets limits on the displayed observed intensity or transition-probability parameters.
The user should also be apprehensive about the precision of the displayed Ritz wavelengths, which is very approximate. It is determined by estimated uncertainty of the
wave number, which is calculated as a combination in quadrature of the level uncertainties. If the latter are unknown, they are estimated as 10 units of the last
significant digit of the level value. Even if they are known, presence of correlations or unknown systematic errors in level values may cause the number of significant
digits in the displayed Ritz wavelength to be wrong by up to ±2. Particularly, for intersystem transitions having different unknown additive constants in the
lower and upper energy levels (e.g., "+x" in one level and "+y" or no additive costant in another level), the wavenumber uncertainty calculated in
this simplistic way may be severely underestimated.
The Ritz wavelengths that have not been evaluated for their precision and stored in ASD are appended with a letter "R". Their presence in the output list of ASD
does not necessarily mean that these transitions can possibly be observed. Many of them are strongly forbidden by additional selection rules that are generally not strict.
E.g., when coupling of electrons is very pure (that is, there is little or no mixing in the eigenvector compositions of the levels), E1 transitions involving a
simultaneous change in more than one electronic subshell cannot occur. An example is a 1s2p-3s2 transition in light He-like ions. Also, when LS
coupling is very pure (such as in light atoms and ions), intercombination transitions (that is, transitions between the levels of different multiplicity) are strongly
forbidden and may not be observable.
This section describes the output for different types of requests from the Lines Search Form:
The output on the screen is HTML-formatted by default, but a significantly
faster ASCII format may also be selected. The output will be even faster and more suitable
for saving and viewing in other software, such as Excel or other spreadsheet viewers,
if the No Javascript box is checked in the Optional Search Criteria section
in the Advanced Settings block of the Lines Input Form.
This box is automatically checked when the user selects the ASCII format. However, it can be unchecked
if the output is intended solely for browsing purposes. The links to the online content of the
bibliographic references are included in the output only if the No Javascript box is not checked.
Help popup windows
The output may contain some symbols or combinations thereof colored
in red. This means that moving a mouse over such symbols would result
in appearance of a small popup window showing some explanatory text
provided the Javascript language is enabled in the browser options or
preferences. Moving the mouse out would remove the popup window unless a user
clicked on the red symbols. In that case, the popup window remains
visible until the next mouse click on the same symbols. For the Ritz
wavelengths, such popup windows appear also for the brown asterisk and pink
plus symbols after the Ritz wavelengths (see below).
 Explanation of the Lines Tables
Explanation of the Lines Tables
(By Column Heading)
This column contains the spectrum name containing an element symbol and a
Roman numeral denoting the spectrum number (I for neutral atom, II for singly
ionized, etc.). This column appears only if multiple spectra have been
specified in the Lines Form input.
The Ritz wavelengths are the wavelengths derived from the lower and upper
levels of the transitions. They are available only if both levels of the
transition are known. If they are available, they usually are more accurate
than the observed wavelengths, especially in the vacuum ultraviolet spectral
region. The accuracy of the Ritz wavelengths depends on the quality of the
energy level values. In some cases, the observed wavelength may be more
accurate than the Ritz one, which is indicated by the number of given
significant figures or by the uncertainties.
The user may choose to display both Ritz and observed wavelengths.
The Obs-Ritz value may also be displayed. By default,
wavelengths are given for vacuum wavelengths below 200 nm and above
2000 nm, with standard-air wavelengths in between. Conversion between the air and vacuum wavelengths
used in ASD is explained here.
Uncertainties of the data can be displayed, if they are available in ASD,
when the corresponding box is checked in the input form (it is checked by default).
If such information is not available, the implied uncertainty is defined by the
number of significant figures given: it is generally between 2.5 and 25 units of the
least significant figure. However, there are exceptions to this rule. In many cases,
uncertainties of the relative positions of spectral lines can be measured more accurately than the
wavelengths or wavenumbers. This may require additional significant figures in the
wavelength values to avoid loss of accuracy. The quality of the Ritz wavelengths can also be assessed
by estimating the average deviation "Obs.-Ritz" for the given
spectral region. Sometimes, the uncertainties of both observed and Ritz
wavelengths can be retrieved from the bibliographic references provided for
each line or from the Primary Data Source references at the top of the output page.
The user can always choose the best available wavelength
from the ASD output.
All uncertainties given in ASD are meant to be on the level of one standard deviation.
There are two options for displaying wavenumbers in the output page:
- The "Wavenumber (all wavelengths)" radio button can be selected in the Additional Criteria section of the
Advanced Settings block of the Lines Search Form.
In this case, instead of the observed and Ritz wavelengths and their uncertainties, the wavenumbers and their
uncertainties will be displayed, and the "Obs-Ritz" column will contain the differences between the observed
and Ritz wavenumbers.
- The "Wavenumber" box can be checked in the Additional Criteria section of the
Advanced Settings block of the Lines Search Form.
This option will have an effect only if the "Wavenumber (all wavelengths)" was not selected (see above).
An additional column containing the wavenumbers will be included in the output table to provide a quick reference to
the wavenumber scale corresponding to the observed and Ritz wavelengths. For lines that have an observed wavelength,
the value given in this column is the observed wavenumber as it is stored in ASD. In some spectra, these values are
directly quoted from the literature sources for the corresponding lines, and their precision may be greater than that
of the observed wavelengths. However, in most cases they were calculated from the observed wavelengths (using the
standard air to vacuum conversion where appropriate) and rounded according to the "rule of 25."
For lines that do not have an observed wavelength, the value given is the Ritz wavenumber, and it is shown in italics.
The Ritz wavenumbers in the ASD output are always calculated from the energy levels stored in ASD.
The number of significant figures in the ASD output values is determined by two considerations:
- Uncertainty of the value should be no less than 2.5 units of the least significant figure of the value. This is
dictated by statistical properties of rounded decimal numbers. All uncertainties given in ASD are meant to be on the
level of one standard deviation.
- The given energy levels and wavelengths should be internally consistent.
About 90 % of data stored in ASD conforms to the rule of 25: the uncertainty is between 2.5 and 25 units
of the least significant figure. Most of the exceptions are of the three origins:
- Legacy data.
Some early researchers rounded their measured data too harshly with the intention of giving the data thought to be
valid to the last given figure. Besides the fact that it is impossible in principle (there is always a finite probability
that the rounded value contains an incorrect last digit), such rounding precludes the use of the results in rigorous
statistical analyses.
- Systematic errors in measurements.
In some cases, absolute positions of measured wavelengths contain poorly known systematic shifts and thus are of poor
accuracy, but separations between them could be measured much more accurately. This requires giving additional figures in the
wavelength values to avoid loss of accuracy.
- Theoretical data.
It is especially difficult to estimate uncertainties of purely theoretical data. Systematic errors caused by approximations
used in the calculation may lead to poor absolute accuracy, but the intervals between the calculated energy levels
may be much more accurate. In such cases, we may give more significant figures than justified by the total uncertainty.
This concerns the Ritz wavelengths derived from purely theoretical levels.
More details are given below for the number of significant figures given for observed and Ritz wavelength and wavenumbers
and their uncertainties.
 Wavelengths
Wavelengths
Precision of the given wavelengths is governed by their uncertainties, so we start with the determination of the uncertainty.
If the uncertainty has been critically evaluated and is stored in ASD, we use this stored uncertainty value. Otherwise, we estimate
the uncertainty of an observed wavelength as ten units of the least significant figure of the wavelength. For Ritz wavelengths with
no critically evaluated uncertainty, we start with determining the uncertainties of the energy levels. If their critically evaluated
values are available in ASD, we use them; otherwise, we estimate them as ten units of the least significant figure of the energy.
Then we combine the level uncertainties in quadrature to obtain an estimated uncertainty of the Ritz wavenumber. This is used to
derive the estimated uncertainty of the Ritz wavelength.
When there is no stored Ritz wavelength value in ASD, it is calculated online from the available
energies of the lower and upper levels, and either an asterisk "*" or a plus "+"
is appended to the Ritz wavelength value. The former simply indicates that
this value was calculated online, while the latter points out that there are no critically evaluated values of the level uncertainties
in ASD, and their estimation involves a number of trailing zeros in the stored energies, which were presumed insignificant. Therefore,
the actual accuracy of the Ritz wavelengths followed by "+" may be higher. This involves only the energy values that
do not contain a decimal point, usually in highly ionized atoms.
The subsequent procedure depends on the type of wavelength to be displayed:
in vacuum or air.
- If the wavelength is to be displayed in vacuum,
- Observed wavelengths are displayed with the same relative precision as they are stored, regardless of the uncertainty.
The latter is given with the same precision as the wavelength.
- Ritz wavelengths are always calculated from the energy levels. If we have a stored critically evaluated value of the
Ritz wavelength, its precision is preserved in the output. Otherwise, it is rounded according to the rule of 25
using the stored or estimated uncertainty value. Estimated uncertainties are not displayed in ASD, as they are not
critically evaluated. Critically evaluated uncertainties are displayed with the same precision as the Ritz wavelengths.
Precision of the stored Ritz wavelengths overrides the rule of 25 for the following reason:
There are many cases when the energy levels derived from observed wavelengths are strongly correlated, i.e., the intervals
between them are known more accurately than the energy levels themselves (which are defined as separations from the ground
level). In such cases, more significant figures may be required to adequately represent the data precision.
- If the wavelength is to be displayed in air,
- If the uncertainty of the air refractive index δn/n is less than 10 % of
the relative uncertainty of the wavelength, the wavelength uncertainty is not modified, and the precision of the output
values is determined by the same rule as for vacuum wavelengths (see above).
- Otherwise, the relative uncertainty of the wavelength δλ/λ is combined in quadrature
with δn/n. If the latter is smaller than half of the wavelength uncertainty stored in ASD, the number
of significant figures in both the wavelength and its increased uncertainty will be preserved as stored in ASD.
Otherwise, both are rounded according to rule of 25. ASD displays critically evaluated uncertainties only.
 Wavenumbers
Wavenumbers
Observed wavenumbers stored in ASD are always shown as is, in units of cm−1.
They may differ from the values derived from the observed wavelengths by a few units of the least significant
figure due to rounding errors. If there is no stored value in ASD, but there is an observed wavelength, the observed
wavenumber is calculated from the wavelength, and its value is rounded according to the rule of 25 using
the stored or estimated uncertainty of the observed wavelength.
The Ritz wavenumbers are calculated from the energy levels stored in ASD. Their precision is entirely
determined by their uncertainties, either stored in ASD (if they were critically evaluated) or estimated from
the uncertainties of the energy levels by combining in quadrature the uncertainties of the lower and upper level
of the transition. If there are no stored critically evaluated uncertainties of the levels, they are estimated
as ten units of the least significant figure of the level value. Then the wavenumber value is rounded according
to the rule of 25.
"Ritz" wavenumbers are derived from level energies
via the Ritz principle: the wavenumber σ of the emitted or absorbed photon is equal to the difference between the
upper and lower energies Ek and Ei,
σ = Ek − Ei.
The Ritz wavelength λ in vacuum is equal to the inverse of σ. If σ is in units of
cm−1, and λ is in nanometers,
λvac [nm] = 107/(σ [cm−1]).
Wavelengths in air are decreased by the refractive index of air.
A numerical value given in decimal representation is considered to be properly rounded if its
uncertainty in the unit of the least significant figure is between 2.5 and 25.
If the rounding is too harsh, i.e., the uncertainty is smaller than 2.5 units of
the least significant figure, the value of the uncertainty is statistical meaningless and the rounded value cannot be used in rigorous
statistical analyses. See more about it in
A. E. Kramida, Comput. Phys. Commun. 182, 419–434 (2011).
If the rounding is too mild, i.e., the uncertainty is greater than 2.5 units of
the least significant figure, the precision of the value cannot be used to estimate the uncertainty. In cases when there are strong
correlations between the given values, mild rounding may be needed to adequately represent the relative uncertainties of
the presented data.
In vacuum, the wavelength λvac is directly determined by the wavenumber σ
(see above). In air, it is decreased by the refractive index n:
In ASD, the index of refraction of air is derived from the five-parameter formula given by
E.R. Peck and K. Reeder, J. Opt. Soc. Am. 62, 958 (1972).
These authors fitted data between 185 nm and 1700 nm.
The conversion between air and vacuum wavelength entails an ambiguity near the
boundary of the air region. For example, a wavelength of 200.0648 nm in vacuum
corresponds to 200.0000 nm in "standard air" (i.e., 15 °C,
101 325 Pa pressure, with 0.033 % CO2).
Conversely, an air wavelength of 199.9352 &nm corresponds to
200.0000 nm in vacuum. In this database, as the default, the
following convention is adopted in terms of the energy difference or
wavenumber, σ=Ek-Ei:
| For σ ≥ 50,000 cm−1 |
→ vacuum wavelengths, |
| For 5000 cm−1 < σ < 50,000 cm−1 |
→ air wavelengths, |
| For σ ≤ 5000 cm−1 |
→ vacuum wavelengths. |
Thus, if the tabulated wavelength lies within
200 ± 0.0648 nm, one must
check the energy difference to ascertain whether it is for vacuum or air.
The relative uncertainty δλ/λ of the formula of Peck & Reeder is 5×10−9 for
wavelengths λ > 400 nm and increases according to the approximate formula
δλ/λ ≈ (0.35734 + 38.24/(λ − 180.29) + 0.000023λ)×10−8
for shorter wavelengths (λ is in nm). The maximum uncertainty is about 9×10−8 at 185 nm.
It should be noted that a number of other formulas for the refractive index of air exist in the literature.
In particular, the formulas given by K. P. Birch and M. J. Downs,
Metrologia 31, 315–316 (1994) have a five times lower
uncertainty than the formula of Peck & Reeder, but only in a restricted wavelength range from 350 nm to
650 nm. Their validity in the extended wavelength region covered by the Peck & Reeder formula has never been verified.
Similarly, the "corrected" formulas given by P. E. Ciddor,
Appl. Opt. 35, 1566–1573 (1996) are valid only in the
wavelength range from 350 nm to 1200 nm and give increasingly large errors for shorter wavelengths: about
−1.0×10−8 at 228 nm and −1.6×10−6 at 185 nm.
 Relative Intensity
Relative Intensity
Relative intensities are source dependent and typically are useful
only as guidelines for low density sources.
These are values intended to represent the strengths of the lines of a spectrum as
they would appear in emission. The values in the Database are taken from the cited publications.
Usually, they are not normalized in any way. In some cases, the intensity values were derived from observed
photometric signals. This would be true for spectra measured by Fourier transform spectroscopy or
in special cases where spectra were recorded photometrically. However, in most cases the values
represent blackening of photographic emulsions used to record an observed spectrum.
These values can be semi-quantitative in that the transmission of the blackened emulsion was
quantitatively measured and used to determine the intensity values.
In other cases, the blackening was estimated visually and the estimates were used for the intensity values.
Thus, the values can range from being approximately quantitative to only qualitative.
Since the Database does not contain information on the origin of the relative intensities,
the relative intensities should be considered as qualitative values that
describe the appearance of a particular spectrum in emission.
The following points should be kept in mind when using the relative intensities:
- There is no common scale for relative intensities. The values in the database are taken from
the values given by the authors of the cited publications. Since different authors use different
scales, the relative intensities have meaning only within a given spectrum; that is, within the
spectrum of a given element in a given stage of ionization.
- The relative intensities are most useful in comparing strengths of spectral lines that are not
separated widely. This results from the fact that most relative intensities are not corrected for
spectral sensitivity of the measuring instruments (spectrometers, photomultipliers,
photographic emulsions).
- The relative intensities for a spectrum depend on the light source used for the excitation.
These values can change from source to source, and this is another reason to regard the values as
being only qualitative.
Descriptors to the relative intensities have the following meaning:
* Intensity is shared by several lines (typically, for multiply classified lines).
: Observed value given is actually the rounded Ritz value, e.g., Ar IV, λ = 443.40 Å.
- Somewhat lower intensity than the value given.
a Observed in absorption.
b Band head.
bl Blended with another line that may affect the wavelength and intensity.
B Line or feature having large width due to autoionization broadening.
c Complex line.
d Diffuse line.
D Double line.
E Broad due to overexposure in the quoted reference
f Forbidden line.
g Transition involving a level of the ground term.
G Line position roughly estimated.
H Very hazy line.
h Hazy line (same as "diffuse").
hfs Line has hyperfine structure.
i Identification uncertain.
j Wavelength smoothed along isoelectronic sequence.
l Shaded to longer wavelengths; NB: This may look like a "one" at the end
of the number!
m Masked by another line (no wavelength measurement).
p Perturbed by a close line. Both wavelength and intensity may be affected.
q Asymmetric line.
r Easily reversed line.
s Shaded to shorter wavelengths.
t Tentatively classified line.
u Unresolved from a close line.
w Wide line.
x Extrapolated wavelength
Other characters occasionally appearing in the intensity column are explained in the quoted literature.
The difficulty of obtaining reliable relative intensities can be understood from the fact that in optically thin
plasmas the intensity of a spectral line is proportional to:
Iik 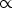 NkAkihνik,
NkAkihνik,
where Nk is the number of atoms in the upper level k
(population of the upper level), Aki is the transition
probability for transitions from upper level k to lower level i,
and hνik is the photon
energy (or the energy difference between the upper level and lower level).
Although both Aki and νik are well defined quantities for each line of a given
atom, the population values Nk depend on plasma conditions in
a given light source, and they are thus different for different sources.
Either transition probability "Aki"
weighted transition probability "gkAki"
(s−1 or 108 s−1), absorption oscillator strength or
f value ("fik"), line strength "S", or "log(gf)" can be
displayed.
Note that fik, S, and
log(gf) are not displayed by default. Also note that
log(gf) is shorthand for
log10(gi fik).
- Aki represents the emission transition probability. In the following formulas, it is assumed to be in units of
108 sec−1.
- fik is the absorption oscillator strength or f-value.
- fik = Aki ·1.49919·10−16
gk/gi λ2, for all multipole types,
where λ is the wavelength in ångströms
- log(gf) is the log10(gi fik),
where gi = 2Ji + 1.
- S is the line strength. It is the electric dipole matrix element squared and
is independent of the transition wavelength.
- More details on these quantities can be found in
this review.
**************************
An estimated accuracy is listed for each transition strength, indicated by a code letter
as given in the table below:
| AAA |
≤ |
0.3% |
| AA |
≤ |
1% |
| A+ |
≤ |
2% |
| A |
≤ |
3% |
| B+ |
≤ |
7% |
| B |
≤ |
10% |
| C+ |
≤ |
18% |
| C |
≤ |
25% |
| D+ |
≤ |
40% |
| D |
≤ |
50% |
| E |
> |
50%. |
The uncertainties are obtained from critical assessments, and
in general, reflect estimates of predominantly systematic
effects discussed in the NIST critical compilations, cited in the
Bibliography.
Accuracies are not available for values listed in the CRC handbook.
If the accuracy is followed by a prime
(′), then a multiplet in the original
compilation has been separated into its component lines and the transition
probability was derived from the compiled value assuming pure LS coupling.
This may decrease the listed accuracy, especially for weaker transitions.
**************************
Transition multipole: Multiply Aki by listed factor to get S:
E1 Electric dipole 4.935525·10−19 gk λ3
M1 Magnetic dipole 3.707342·10−14 gk λ3
E2 Electric quadrupole 8.928970·10−19 gk λ5
M2 Magnetic quadrupole 6.707037·10−14 gk λ5
E3 Electric octupole 3.180240·10−18 gk λ7
M3 Magnetic octupole 2.388852·10−13 gk λ7
where λ is the wavelength in angstroms and gk is
the statistical weight of the upper level. The numerical
factor for the electric quadrupole conversion from Aki to S follows
a more modern convention than that used in the original publications, which
will be used in future NIST publications.
Lower level and upper level energies of the transition are displayed in the
units specified. Note that, if the units other than cm−1 are requested in the
Lines Search Form, uncertainties of the conversion factors
are combined in quadrature with the uncertainties of the energy levels, which may result in loss of precision. However,
the Ritz wavelengths and wavenumbers are always calculated from the stored energy levels in units of
cm−1, so their precision is not affected.
If the "No Javascript" checkbox is not checked, the level energies appear in the output as active links, even if the ASCII format is
chosen. Clicking on such an active level-value link will open a new tab containing a list of all lines originating from or terminating
on this level.
Configurations of the lower and upper levels are displayed.
For ASCII output, periods are inserted in the configuration labels whenever
necessary to avoid ambiguity due to the lack of superscripts, and angular
brackets enclose J values of the parent term.
Terms of the lower and upper levels are displayed.
A superscript "°" in the HTML output or an asterisk in the ASCII output indicates odd parity.
The J-values represent
the total electronic angular momentum of the lower and upper levels.
gi-gk
represents statistical weight of the lower level (gi=2Ji+1) and
statistical weight of the upper level (gk=2Jk+1).
This filed is blank for allowed (electric-dipole, or E1) transitions, including the
spin-changing (intercombination) transitions. Types of forbidden transitions
are denoted as follows:
M1 - Magnetic dipole.
E2 - Electric quadrupole.
M2 - Magnetic quadrupole.
E3 - Electric octupole.
M3 - Magnetic octupole.
M1+E2 - Mixed magnetic dipole and electric quadrupole transition.
The selection rules for these two types of transitions are the same, so
both types of transitions can contribute to the observed intensity and
radiative rate Aki. The line strength is undefined for such mixed transitions.
2P - Two-photon transition.
HF - Hyperfine-induced transition (may occur only in isotopes with a
non-zero nuclear magnetic moment).
UT - Forbidden transition of an unspecified type.
Transitions that are strongly forbidden in isolated atoms may be enabled by
environmental effects such as external electric or magnetic fields. Such
forbidden transitions would be denoted as type = UT in ASD.
The conversion formulas between transition probabilities and line strengths for different types of allowed and forbidden
transitions are given here.
There may be several reference codes separated by comma in each of these columns.
If the "No Javascript" box was not checked in the Lines Search Form, each
bibliographic code links to a popup window showing a bibliographic reference for transition probability or for the observed spectral
line.
For those lines that have diagnostic data in ASD, an additional column of the Lines Tabular Output
contains links to detailed information about the line pairs whose relative intensities can be used
to determine the plasma temperature and/or density. The diagnostic data include
plots of intensity ratios versus electron temperature or density and specifications of
the validity range of these plots.
Line Identification Plot
If the "Line Identification Plot" option has been selected on the Lines Form,
two links will appear at the very bottom of the tabular output page,
i.e., a link to a PDF file containing an image of the plot and a link to a new popup window displaying the wavelengths of
the spectral lines shown on the PDF plot.
The Line Identification Plot is a stick plot showing the line positions for all chosen
ions in the given wavelength range. It can be used to diagnose which ions produce spectral lines is an observed spectrum. For that,
the image should be magnified or reduced to approximately the same wavelength scale as in the experimental spectrum. Then the
patterns of intervals between the observed spectral lines could be matched with those in one or more of the ion spectra in the
Line Identification Plot, which can help the user to identify the observed lines.
If there are too many lines in the chosen spectra, the user can use the "Relative intensity minimum" or other
options in the Optional Search Criteria section in the Advanced Settings
block of the Lines Input Form to reduce the number of shown lines.
Spectrum Image Plots
These plots are colored visualizations of simulated spectra. They are displayed as an image at the bottom of the lines tabular output.
See their description here.
Saha-LTE Spectrum Plots
The Saha-LTE plot shows the distribution of calculated intensities in the plasma emission spectrum for the chosen ions within
the selected wavelength range.
If the "Saha-LTE Plot" option has been selected on the Lines Form, two or more links will appear at the very bottom of the
tabular output page. One of them is a link to a PDF file containing an image of the plot. One of the
other links with text "Relative Line Intensities" is to a new popup window displaying the wavelengths
of the spectral lines shown on the PDF plot and their relative intensities. If the user requested a Doppler-broadened spectrum
(by checking the corresponding box in the Lines Form), there will be two additional links, one to table of calculated intensities of each ion
in the Doppler-broadened spectrum, and one to a table showing the distribution of intensities in the total spectrum (sum of contributions
from each ion). Both these tables are two-column plane fixed-column-width ASCII files with the first column having the wavelength
grid points and the second column having relative intensities in arbitrary units. These tables can be used to plot the spectra
with other software.
The header of the plot shown in the PDF file shows the user-defined parameters: composition of the element mixture in the plasma,
electron temperature and density, as well as the total number of lines in the synthetic spectrum. If a Doppler-broadened
spectrum was requested, the header also shows the ion temperature used to calculate the Doppler broadening. It may be greater
than the Ion Temperature specified by the user in the Lines Input Form, if the number of required
points in the grid was too large for the given selection of spectral lines. In such cases, to display narrower lines (corresponding to
a lower ion temperature), the user needs to reduce the selected wavelength range, reduce the number
of requested spectra, or use some line-filtering options in the
Optional Search Criteria section in the Advanced Settings
block of the Lines Input Form.
If higher resolution (narrower lines) is needed, and only neutral, singly-ionized, or doubly-ionized atoms are involved,
consider using the LIBS Interface of ASD. Although this interface does not allow drawing spectra
for a particular ionization stage, its plots can be calculated on the user's computer with much higher resolution, and they have
flexible interactive features.
Working with the Grotrian diagram plot
Basically, only a computer mouse and a space bar are used for interaction with this plot.
- Default view
Initially all levels and transitions are shown on the Grotrian Diagram (GD)
plot. Each energy level is shown by a horizontal bar. The colors
(black and blue) have no meaning and are used simply to help with
visualization of the plot. The X-axis corresponds to different level
series, and the Y-axis shows the level energy in cm−1. The
radiative transitions between the levels are shown as slanted gray lines.
The ionization limits are shown as magenta horizontal lines. At the
top of the plot, the total number of levels, lines and ionization
limits is displayed. These parameters are automatically updated when
zooming in or out. In the bottom right part of the plot, the maximum
and minimum values of the transition probability for the displayed
lines are given in the input text fields. The "Submit" and "Reset"
buttons are used for setting the limits for transition probabilities,
while the "Isolate" button is used to single out one level and all
relevant transitions. The green field ("zoom field" below) next to the Y-axis is used for
zooming in and out. Below, the top right part of the plot will be
referred to as the "info field."
- Controls block
The appearance of the GD plot can be changed in many ways by using the various control elements (action buttons,
radio buttons, and input boxes) located in the control block above the GD plot. These control elements are explained below.
- Plot size
A user change the size of the GD plot by clicking on the "Enlarge" or "Shrink" buttons.
- Grouping by configuration, subconfiguration, or J values
By default, the levels are grouped into series according to their electronic-shell configuration.
For instance, in O I the levels belonging to configurations 2s22p3nl
(l = s, p, d, f, g) or 2s2p4np are assigned to different series,
each displayed in a separate vertical column in the plot. However, there are different core configurations in all these series,
and stronger transitions tend to occur between the series having the same core. To better see the connections between the series based on different cores,
the user can choose grooping of levels by subconfigurations. In this case, the
2s22p3(4S°)nl and 2s22p3(2D°)nl levels
would be assigned to different series. For the purpose of seeing connections between the levels having different total orbital momentum J,
the levels can be grouped by J. The choice of level grouping is made in the "Group by" set of radio buttons in the left part
of the control block
- Filtering by J-values, multiplicities, configurations, and subconfigurations
The menus with the corresponding names contain the sets of values available for the chosen spectrum. By default, all these values are included in the
selection of levels displayed in the plot. The user can restrict the number of levels and transitions displayed by selecting a subset of the available
values and then clicking the "Filter lines/levels" button.
For example, selecting only "3" in the "Multiplicities" menu for O I would result in display of only triplet levels and transitions
between them, while selecting only "0" and "1" in the "J values" menu would produce a GD only for levels
having J = 0 and 1 and corresponding transitions. Clicking the button "Reset filters" will restore the default
setting (no filtering).
- Show only radiatively linked levels
By default, all levels belonging to the chosen ion are displayed in the
GD. Selecting the "Radiatively linked" option would result in display of only those levels
that are connected by radiative transitions.
- Filtering A-values
The range of A-values for transitions displayed in the GD can be specified by entering the limiting values in the "min A"
and "max A" input boxes and then clicking the "Filter lines/levels" button. By default, the values in these boxes are 0 and the maximum
available A-value for this spectrum. The zero minimum value implies that transitions with unknown A-values are included in the GD.
Other filtering options, such as displaying only the observed lines, only the allowed or forbidden lines, as well as different units for levels and wavelengths
can be implemented by returning to the Lines Input form and sellecting appropriate options in the Advanced Settings section.
- Selecting, deselecting and cycling over objects
Mouse:
- Clicking on any level colors it and all relevant
transitions in red. In addition, the basic data on this level, e.g.,
energy, configuration, etc., are displayed in the info field.
- Clicking on any line
colors the line and the lower and upper level in red. In addition, the
basic data on the levels and line (wavelength, transition probability,
etc.) are shown in the info field.
- Consecutive clicking on two levels is equivalent to clicking
on the corresponding spectral line. If there is no line connecting the
levels, no Line information is shown in the info field although the
data on both levels are displayed.
- Clicking anywhere in the white background of the GD subwindow
deselects the already selected object(s).
Space bar:
- If no objects have been selected, pressing a space bar
selects a first spectral line in the following order: the levels are
assumed to be arranged first according to the X-axis label (from left
to right) and then according to the level energy. Since the ground
state always has a zero energy, pressing the bar would normally
highlight a line originating from the ground state.
- If a line has been selected, pressing a space bar cycles over
all spectral lines on the plot in the order described above, that is,
(i) for the same lower level, the upper level is updated, and
(ii) after all lines for a particular lower level have been cycled through, the lower
level is updated, and so on.
- If a level has been selected, pressing a space bar cycles over all energy levels within the levels series.
- The data shown in the info field is the same as for the mouse selection.
- Zooming in and out
The green field to the right of the Y-axis is used for zooming in. The
upper and lower limits for the energy levels are set up by clicking the
mouse in the zoom field. The upper and lower limits are shown by
horizontal blue lines. When both limits are selected, the "Zoom" button
above the Y-axis becomes highlighted and clicking on it with the mouse
results in updating the subwindow. This procedure can be repeated infinitely.
After zooming in, the "Reset" button on the above the Y-axis becomes
highlighted, and clicking on it restores the original plot.
- Isolating a level
In order to isolate one energy level with all radiatively connected
levels, one has first to select a level by a mouse click. Then, the
"Isolate" button in the bottom right part of the plot becomes
highlighted, and pressing it would result in displaying the red group
of levels and lines only. If necessary, a user can then perform zoom or
transition probability limiting procedures on this subset of levels.
After isolating a level, the "Isolate" button changes its label to
"Show All," and pressing this new button would show all available
levels and transitions.
 Creating an output for importing into a spreadsheet
Creating an output for importing into a spreadsheet
Two options are available from the Format Output pull-down menu for creating an output in a format suitable for
importing into a spreadsheet: CSV (comma-separated ASCII file) or tab-delimited ASCII file. Both options produce output in the user's
browser window, which needs to be saved on the local computer by using the browser's interface options and opened with a spreadsheet software.
For importing data into Excel, the most convenient format is CSV. If the output is saved in a file with a .csv extension, some browsers
(e.g., Chrome) even allow the user to open it directly from the browser. Users of the OpenOffice software may prefer the tab-delimited format,
as it conveniently allows setting the format of all columns enclosed in double quotes as "text." This is required, for example, for
the J-values, which can be half-integers stored as strings, e.g., "1/2." If such columns are not set as text, the spreadsheet software
will most likely automatically convert them to dates. Even for columns containing only numbers, text format is needed to preserve the number of
decimal places, which indicates an implicit uncertainty of value. For this reason, the CSV-format output contains formulas instead of plain values.
After importing such a file into a spreadsheet, the user should "select all cells" (in Excel, this can be done by clicking on the small triangle
in the upper left corner of the spreadsheet), copy the selection, and paste it back as values.


 Spectral Lines
Spectral Lines Lines Search Form
Lines Search Form Main Search Parameters
Main Search Parameters Selecting Spectra for Line Searches
Selecting Spectra for Line Searches
 Lower and upper limits of the wavelength/wavenumber/photon energy/frequency range
Lower and upper limits of the wavelength/wavenumber/photon energy/frequency range
 Graphical Output Options
Graphical Output Options
 Dynamic Plot Options
Dynamic Plot Options
 Java Grotrian Diagrams
Java Grotrian Diagrams
 Advanced Settings
Advanced Settings
 Optional Search Criteria
Optional Search Criteria
 Additional Search Criteria
Additional Search Criteria
 Displaying Ritz Wavelengths of All Possible Electric Dipole Transitions
Displaying Ritz Wavelengths of All Possible Electric Dipole Transitions Lines Output
Lines Output Output Line Tables
Output Line Tables Explanation of the Lines Tables
Explanation of the Lines Tables Ion
Ion Uncertainties of Observed and Ritz Wavelengths
Uncertainties of Observed and Ritz Wavelengths Significant Figures
Significant Figures Wavelengths
Wavelengths Wavenumbers
Wavenumbers Conversion between air and vacuum wavelengths
Conversion between air and vacuum wavelengths Relative Intensity
Relative Intensity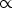 NkAkihνik,
NkAkihνik,
 Transition Strengths
Transition Strengths Accuracy
Accuracy Conversion between transition probabilities and line strengths
Conversion between transition probabilities and line strengths Ei-Ek
Ei-Ek TP Ref. and Line Ref.
TP Ref. and Line Ref. Graphical Output
Graphical Output Plotting Grotrian diagrams
Plotting Grotrian diagrams Creating an output for importing into a spreadsheet
Creating an output for importing into a spreadsheet